Except where otherwise noted, this work is distributed under the terms of a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International licence (CC BY-NC-ND), a copy of which is available at http://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Summary
What Did We Study?
- The real-world use of glucagon-like peptide-1 (GLP-1) receptor agonists (RAs), which are funded for type 2 diabetes mellitus (T2DM) across public drug plans in Canada, was analyzed to determine their current utilization patterns and estimate their suspected use outside of T2DM.
- We measured total expenditures by payers, how GLP-1 RAs were being used in combination with other drugs for T2DM (proportion of claimants on different drug regimens), and the suspected use of GLP-1 RAs outside of T2DM (proportion of claimants using GLP-1 RAs without a history of using diabetes drugs or glucose monitoring devices).
Why Did We Study GLP-1 RAs?
- Drug plan expenditures in this drug class have increased significantly in recent months (correlated with increased activity in regulatory approvals and direct-to-consumer advertising) and we wanted to assess the extent of the utilization that was “off reimbursement criteria” (i.e., use outside of T2DM) because these drugs have demonstrated efficacy in other conditions that are not currently publicly funded but have regulatory approval (e.g., weight management).
How Did We Study GLP-1 RAs?
- This analysis was conducted using data from provincial and territorial (PT) public drug plans and non-formulary claims (claims outside those reimbursed by PT drug plans such as federal drug plans, cash pay, and private insurance) from British Columbia, Manitoba, and Saskatchewan for calendar years 2019 to 2021.
- CADTH partnered with the Canadian Institute for Health Information (CIHI) in a pilot project to collect and assess real-world drug claims data to describe claimants and calculate expenditures for GLP-1 RAs to estimate the proportion of monotherapy versus combination therapy use and use in T2DM versus suspected use outside of public drug plan criteria (i.e., non-T2DM).
What Did We Learn?
- Ozempic (semaglutide injection) is the dominant GLP-1 RA brand (> 99% market share among public PT drug plans) and expenditures on it have accelerated. Expenditures on Ozempic have increased from $13.5 million in 2019 to $227 million in 2021.
- There may be some patient populations among PT drug plans who are not using Ozempic appropriately in T2DM. This may include a portion of the 17% who used it as a monotherapy and the 12% who used it in combination with dipeptidyl peptidase-4 inhibitors (data from 2021).
- Increasing use of Ozempic can be partially attributed to non-T2DM claims. The proportion of claimants with suspected use outside of T2DM was 15% in Ontario and ranged from 0% to 8% across the other PT public drug plans; suspected use outside of T2DM is projected to be 1 in 5 claimants in Ontario in 2022. Among non-formulary claims (e.g., federal public plans, private insurance), this proportion ranged from 36% to 74% (data from 2021).
What Does This Mean?
- The expenditure on Ozempic is rapidly increasing, and Ozempic’s suspected use outside of T2DM appears to be significant in drug plans that employ a less restrictive listing status (i.e., general benefit) or less restrictive reimbursement criteria.
- Formulary management strategies can be applied to promote the appropriate use of GLP-1 RAs, such as requiring prior authorization or conducting prescriber audits. Future research in this field is warranted to assess if similar patterns are seen with private insurance claims, if the use of GLP-1 RAs outside of T2DM can be detected for individuals circumventing current prescribing criteria, and to study the effects of direct-to-consumer advertising on appropriate utilization of medications.
Background
Type 2 Diabetes Mellitus
Diabetes mellitus (DM) is estimated to affect approximately 8.8% of all people living in Canada (9.4% of men, 8.1% of women, aged 1 year or older) based on the latest (2019) statistics from the National Diabetes Surveillance System.1 Although the age-standardized incidence rate remained relatively stable from 2000 to 2019, the age-standardized prevalence rate of DM increased by 3.3% per year and all-cause mortality rates among individuals living with DM decreased by 2.1% per year.1 Type 2 DM (T2DM) is 10 times more common than type 1 DM — 90% of individuals with DM in Canada have T2DM. T2DM is known to be affected by several lifestyle, social, biological, and genetic factors, including obesity, intake of processed foods, physical inactivity, lower socioeconomic status, increased age, and race or ethnicity.1,2 Metformin (MET) is considered first-line treatment as an oral glucose-lowering agent; if MET monotherapy and diet and lifestyle modifications are insufficient to achieve adequate glycemic management, a second or third oral antidiabetic drug (e.g., sulfonylureas, meglitinides, thiazolidinediones alpha-glucosidase inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium-glucose cotransporter-2 [SGLT2] inhibitors) or progression onto injectables (i.e., insulin or glucagon-like peptide-1 [GLP-1] receptor agonists [RAs]) may be added as needed.3
Reports from the Framingham Heart Study showed that T2DM independently increases the risk of cardiovascular disease and mortality, primarily through either myocardial infarction or heart failure.4 In addition, evidence from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial associated increased rates of cardiovascular-related death with intensive glucose-lowering.5 Following directives from health regulatory agencies (e.g., US FDA in 2008) to measure cardiovascular outcomes in T2DM clinical trials, it was observed that some newer antidiabetic drugs tested at the time provided improved cardiovascular outcomes in individuals living with T2DM.6 In particular, GLP-1 RAs and SGLT2 inhibitors showed promise, with glucose-lowering and cardioprotective properties as well as renal benefits.7
Glucagon-Like Peptide-1 Receptor Agonists
GLP-1 RAs are a group of antidiabetic drugs that act as glucose-lowering agents via the stimulation of GLP-1 receptors, G-protein–coupled receptors that potentiate insulin secretion from pancreatic islet beta cells.8 Six GLP-1 RAs have been approved by Health Canada for the improvement of glycemic management in adults living with T2DM, either as an adjunct therapy to diet and exercise or in combination with other antidiabetic drugs in addition to diet and exercise if adequate glycemic management with other antidiabetic drugs plus diet and exercise has not been achieved (Appendix 1).9-15 All GLP-1 RAs are administered by subcutaneous (SC) injection, except 1 oral formulation of semaglutide (Rybelsus). GLP-1 RAs also have other non-glycemic properties related to appetite suppression and weight loss that are beneficial in T2DM16,17; 2 of these GLP-1 RAs, liraglutide (Saxenda) and semaglutide (Wegovy), are also approved in Canada for weight management.18,19
GLP-1 RAs in T2DM and Weight Management
Due to their additional effects on and recent approval from Health Canada for weight management (for Saxenda and Wegovy),18-22 GLP-1 RAs were recently submitted for CADTH Canadian Drug Expert Committee (CDEC) review for weight management(Appendix 2).23,24 Saxenda received a “do not reimburse” recommendation from CDEC in September 2021.23 Wegovy was submitted for CDEC review in March 2022, and was reviewed during the July 2022 CDEC meeting; final recommendations from CDEC are still pending.24
Given the prevalence of obesity in Canada (approximately 1 in 4 adults),25 the potential use of GLP-1 RAs for weight management would have a significant impact on drug plan spending if reimbursed. Because the T2DM formulations of these drugs are already on the Canadian market, it is possible that physicians may already be prescribing these medications to individuals for weight management (i.e., not in accordance with the intended drug plan reimbursement criteria). Therefore, there was rationale to assess the use of GLP-1 RAs across Canada to understand the current utilization of and expenditures on these medications in the country.
GLP-1 RAs and Direct-to-Consumer Advertising
The role of social media in driving consumers to demand the use of Ozempic for weight loss was recently highlighted as contributing to drug shortages in Australia.26,27 TikTok was cited as a driving factor for the increased consumer demand for Ozempic for weight loss, based on the use of hashtags such as #ozempicjourney, #ozempicaustralia, or #ozempicchallenge. Videos with these hashtags documenting weight loss had amassed as many as 74 million total views as of May 2022. The consequences of shortages of Ozempic for individuals living with T2DM can be severe, and a Joint Statement by the Australian Medical Association, the Australian Diabetes Society, the Pharmaceutical Society of Australia, and Novo Nordisk Pharmaceutical called for prioritization of the Ozempic supply for individuals living with T2DM because of an ongoing expected shortage continuing until December 2022.28
In Canada, there is evidence of direct-to-consumer advertising of Ozempic in social media and traditional media. Between October 31, 2014, and December 31, 2021, Health Canada received 6 advertising complaints about Ozempic (and 4 others for Rybelsus and Saxenda) concerning direct-to-consumer advertising of unauthorized claims in radio and television media; these complaints are now considered closed.29
Purpose of This Analysis
The aim of this analysis was to determine the utilization of GLP-1 RAs and assess their drug expenditures, prior and concurrent antidiabetic drug use by GLP-1 RA, and the proportion of claimants potentially using GLP-1 RAs for a suspected non-T2DM indication.
Policy Issues
In Canada, only individuals with T2DM are eligible for reimbursement of GLP-1 RAs. Despite the reimbursement criteria, physicians may already be prescribing these medications to individuals for non-diabetic use (i.e., outside of T2DM or “off-criteria”) because GLP-1 RAs have beneficial effects in addition to glycemic management (e.g., weight management, cardiovascular risk). More specifically, lixisenatide (Adlyxine) and semaglutide (Ozempic) are listed as an open benefit on some public drug plan formularies.30 To help promote the appropriate use of GLP-1 RAs in Canada, we conducted an analysis to gain insights on the utilization of these medications within public drug plans.
Policy Questions
- What are the trends in the utilization patterns of GLP-1 RAs across Canada?
- Are GLP-1 RAs potentially being prescribed for non-diabetic use in Canada?
Research Questions
- What are the reimbursement criteria for GLP-1 RAs within each jurisdiction?
- What were the utilization patterns of GLP-1 RAs nationally and within each jurisdiction from 2016 to 2021?
- What is the estimated frequency of suspected non-diabetic use among GLP-1 RA claimants nationally and within each jurisdiction?
- What proportion of individuals prescribed a GLP-1 RA had no prior claim for another antidiabetic drug or glucose monitoring device (i.e., glucometer with strips, flash glucose monitoring, or continuous glucose monitoring)?
- What proportion of individuals prescribed a GLP-1 RA used it as monotherapy?
- Given that the original dosing for liraglutide and semaglutide differed for weight management (Saxenda and Wegovy) versus T2DM (Victoza and Ozempic) before January 2022, do dosing patterns for the T2DM formulations indicate potential use in weight management?
- What are the trends in GLP-1 RA expenditures nationally and within each jurisdiction from 2016 to 2021?
Methods
Data Sources
To determine the reimbursement criteria for GLP-1 RAs across public drug plans, formulary websites and documents containing lists of regular benefit and restricted access drugs were searched. The reimbursed formulations, criteria, and any restrictions and notes were summarized for all public drug plans except Quebec (Appendix 3). Note Alberta data are available up to Q3 2021.
A request was made to the Canadian Institute for Health Information (CIHI) to extract claims data for GLP-1RAs from the National Prescription Drug Utilization Information System (NPDUIS) database for all public provincial and territorial (PT) drug plans and non-formulary claims (federal drug plan, out-of-pocket, and private insurance claims for British Columbia, Manitoba, and Saskatchewan only), with the exception of Quebec, between January 1, 2016, and December 31, 2021 (Appendix 4). GLP-1 RAs were identified by the Drug Identification Numbers (DINs) assigned by Health Canada and by the WHO Anatomical Therapeutic Chemical (ATC) codes provided by the requestor. The ATCs included in this analysis are presented in Appendix 5.
Claims for drugs administered outside of public drug plans (e.g., through hospital-based programs or cancer agencies) and covered by jurisdictions are not submitted to NPDUIS. Non-formulary drug claims included those reimbursed by federal drug plans, private insurance, or out-of-pocket cash pay. In accordance with the CIHI privacy policy, in cases in which the number of active beneficiaries were less than 5 (but greater than zero), this number and other associated values were suppressed to ensure confidentiality.
The NPDUIS database does not include information regarding any of the following:
- prescriptions that were written but never dispensed
- prescriptions that were dispensed but for which the associated drug costs were not submitted to, or not accepted by, the public drug program
- diagnoses or conditions for which prescriptions were written.
Data Analysis
This section provides brief descriptions of each of the analyses conducted for this report. Additional details on the study design and analysis methods are available in Appendix 6. The data elements provided in the dataset provided by CIHI are defined in Appendix 7.
Reimbursement Criteria
The reimbursement criteria of GLP-1 RAs were tabulated and summarized descriptively.
Utilization Patterns
Utilization patterns were based on the number of total unique claimants of GLP-1 RAs through drug programs by jurisdiction and year from 2016 to 2021. Market shares of GLP-1 RAs were calculated as a proportion of all GLP-1 RA claimants for each jurisdiction and at the national level.
Suspected Non-Diabetic Use of GLP-1 RAs
Suspected non-diabetic use among GLP-1 RA claimants was estimated using 3 different approaches because of the limitations associated with administrative data. The first approach considered prior drug claims history because the indication listed in the product monograph and the reimbursement criteria for GLP-1 RAs are based on previously trialled antidiabetic therapies; prior device claims were also considered in this approach because some jurisdictions reimburse glucose monitoring systems for individuals living with T2DM. The second approach examined the use of GLP-1 RAs as a monotherapy versus their use in combination with other antidiabetic medications because there is a possibility that individuals using a GLP-1 RA as monotherapy are using it for a non-diabetic indication unless they had a contraindication or intolerance to other antidiabetic medications. The third approach considered dosing (for liraglutide and semaglutide only), which was based on the premise that initial dosing regimens for these medications were different for T2DM versus other indications (e.g., the maintenance dose that was approved for the weight management indication was approximately twice as much as the dose initially approved for T2DM). The 3 approaches are:
- Approach 1: The proportion of GLP-1 RA claimants without prior antidiabetic drug or glucose monitoring device claims (Appendix 6) within a 2-year look-back period. Additionally, claimants deemed non-diabetic use using this definition were included in an analysis to determine if they continued to not to make claims for another antidiabetic drug or glucose monitoring device (i.e., continued non-diabetic use) in the look-forward period (i.e., any time after the index GLP-1 RA claim).
- Approach 2: The proportion of GLP-1 RA claimants using the GLP-1 RA as monotherapy (i.e., suspected non-diabetic use unless the patient had a contraindication or intolerance to other antidiabetic drugs).
- Approach 3: The proportion of Victoza and Ozempic claimants using these medications at various dose regimens.
- Before January 2022, the maintenance doses for both Victoza (1.8 mg daily) and Ozempic (1.0 mg weekly) were approximately half that of their weight management formulations (Saxenda: 3.0 mg daily; and Wegovy: 2.4 mg weekly). As of January 2022, a higher maintenance dose (2.0 mg weekly) was approved for Ozempic for T2DM. This analysis categorized GLP-1 RA claimants into different dosing categories considering this.
These analyses were conducted for each drug by year from 2016 to 2021 for each jurisdiction and at the national level. The dosing analysis (approach 3) was conducted using public formulary claims data only as dosing data related to non-formulary claims were not available.
Drug Expenditures
Expenditures were based on the total prescription cost accepted by the drug plan. This is the total dollar amount of a prescription accepted by the drug plan as eligible toward a deductible or for reimbursement, as it relates to the quantity accepted, which includes the drug cost as well as the associated professional fees and markup, if applicable. Total expenditures were calculated for each drug, by year from 2016 to 2021, within each jurisdiction and at the national level. This analysis was conducted using public formulary claims data only because expenditure data related to non-formulary claims were not available.
Findings
Reimbursement Criteria for GLP-1 RAs in T2DM
Lixisenatide (Adlyxine) and semaglutide injection (Ozempic) are the only GLP-1 RAs reimbursed by any Canadian public drug plan formulary (Table 1). The restrictions for reimbursement for Adlyxine and Ozempic concern their usage as add-on treatment in cases in which first-line metformin, and varying combinations of sulfonylureas and/or insulin as add-on treatment, fail to regulate glucose levels in T2DM. The specific criteria for reimbursement of Adlyxine and Ozempic across public drug plans are presented in Appendix 3.
Adlyxine is listed as an open benefit in Ontario and under the Non-Insurance Health Benefits (NIHB) and Veteran Affairs Canada federal plans, and it is reimbursed with restrictions in Alberta, Saskatchewan, Manitoba, New Brunswick, and Prince Edward Island. It is not reimbursed in British Columbia, Nova Scotia, Newfoundland and Labrador, or Yukon, nor by the Canadian Armed Forces or Correctional Service of Canada (CSC) federal plans.
Ozempic has the most extensive listing of any GLP-1 RA; it is reimbursed with restrictions across all public formularies except for Ontario, CSC, NIHB, and Veterans Affairs Canada, where it is listed as Open Benefit. Among public drug plans that do not list Ozempic as open benefit, Alberta and New Brunswick specifically state that eligibility includes a contraindication or intolerance to MET, sulfonylureas, or insulin, whereas the remaining jurisdictions state that only individuals who do not achieve adequate glycemic management with these medications are eligible for Ozempic.
Table 1
Overview of Formulary Listings for GLP-1 RAs in T2DM by Public Drug Plans in Canada.
GLP-1 RA Claimants and Market Shares
Figure 1 presents yearly trends for public PT formulary and non-formulary (British Columbia, Manitoba, and Saskatchewan only) GLP-1 RA claimants from 2016 to 2021. The number of GLP-1 RA claimants across Canada has risen year over year among both public PT and non-formulary drug claims. Among public PT formularies, this number has risen more than 5-fold from 2019 (24,733 claimants) to 2021 (128,910 claimants), with Ozempic dominating the GLP-1 RA market share (almost 100%) since 2019. Among non-formulary claims, the number of GLP-1 RA claimants has increased more than 4-fold from 2016 (14,088 claimants) to 2021 (60,120 claimants). There has been an increase in the use of Ozempic year over year since 2018, and it now represents 69% of the GLP-1 RA market share among non-formulary claimants in 2021. Note that Ozempic was approved by Health Canada in January 201812 and was given a “reimburse with clinical criteria and/or conditions” recommendation by CDEC in May 2019.31
Because this utilization analysis revealed that Ozempic is the dominant GLP-1 RA brand among public PT drug plans in Canada, the following sections focus on the results related to this specific product.

Figure 1
National Number of GLP-1 RA Claimants and Market Shares Across PT Formulary and Non-Formulary Drug Claims in Canada.
Ozempic Utilization by Jurisdiction
Since 2019, most public PT Ozempic claimants have been from Ontario, although this proportion is decreasing year over year (Figure 2). In 2021, 26.7% of Ozempic claimants were from jurisdictions outside of Ontario, whereas this proportion was approximately 11.5% claimants in 2019.

Figure 2
Ozempic Claimants by Jurisdiction.
Suspected Non-Diabetic Use of Ozempic Based on Prior Claims History (Approach 1)
Table 2 presents the percentage of claims for suspected non-diabetic use of Ozempic based on prior claims history (i.e., no prior claim for other antidiabetic drugs or glucose monitoring) across public PT drug plans for the years 2019 to 2021. Overall, the proportion of Ozempic claimants in Canada representing suspected non-diabetic use has increased year over year, more than doubling from 4.2% in 2019 to 10.7% in 2021. In the additional analysis conducted to determine what proportion of these claimants continued to be deemed non-diabetic use in the look-forward period, 70.5% of these claimants were still suspected of non-diabetic use after the index Ozempic claim.
Table 2
Non-Diabetic Use of Ozempic Based on Prior Claims History by Jurisdiction and Year Across Public Provincial and Territorial Formularies (Percentage of All Ozempic Claimants) .
The jurisdictions with the highest proportion of suspected non-diabetic use of Ozempic (percentage of Ozempic claimants) in 2021 were Alberta (5.0%), New Brunswick (8.1%), and Ontario (14.9%). Trends in the suspected non-diabetic use of Ozempic for these 3 jurisdictions and the national figures from 2019 to 2022 (2022 estimates are projected) are provided in Figure 3. Linear projections estimate that the suspected non-diabetic use of Ozempic will continue to rise to approximately 14% nationally in 2022, up from 10.7% in 2021. Among the 3 jurisdictions with the most frequent suspected non-diabetic use of Ozempic, the proportions are projected to increase in 2022 to 19.4% in Ontario, 13.4% in New Brunswick, and 6.9% in Alberta. These findings may be explained by the listing status or reimbursement criteria for Ozempic in these 3 jurisdictions and assume that no formulary management policies would be implemented in 2022 to curb use.

Figure 3
Suspected Non-Diabetic Use of Ozempic Based on Prior Claims History in Ontario, New Brunswick, and Alberta Public Formularies Over Time and Projected to 2022 (Percentage of Ozempic Claimants).
Suspected non-diabetic use of GLP-1 RAs is also prevalent among non-formulary drug claims (although this was expected for Saxenda), more so than what was observed among the PT drug plans (Table 3). For comparison, relative to the 14.9% of Ozempic claimants using the drug for suspected non-diabetic use in Ontario in 2021, this proportion ranged from 35.6% (in Manitoba) to 73.8% (in British Columbia) among the 3 provinces included in the non-formulary claims data. The proportion of suspected non-diabetic use among non-formulary GLP-1 RA claimants has increased year over year, especially with Ozempic, Rybelsus, Trulicity, and Victoza, and will likely continue to rise similar to what is occurring among the PT drug plans.
Table 3
Suspected Non-Diabetic Use of Ozempic Based on Prior Claims History by Jurisdiction and Year in Non-Formulary Claims (Percentage of GLP-1 RA Claimants) .
Ozempic Use as Monotherapy and in Combination Therapy (Approach 2)
Table 4 provides the year-to-year trends in the proportion of Ozempic claimants who were using it as monotherapy (i.e., suspected non-diabetic use unless the patient had a contraindication or intolerance to other antidiabetic drugs) across public PT formularies. Nationally, the proportion of Ozempic monotherapy claimants remained consistent year over year. Similar to the analysis on suspected non-diabetic use based on prior claims history, the provinces of Ontario, New Brunswick, and Alberta represented jurisdictions with the highest percentage of Ozempic monotherapy claimants in 2021 at 17.2%, 16.3%, and 15.5% of Ozempic claimants, respectively, although British Columbia had the highest proportion among all jurisdictions (21.1%).
Table 4
Ozempic Monotherapy Claimants by Jurisdiction and Year Across Public Provincial and Territorial Formularies (Percentage of Ozempic Claimants).
GLP-1 RA monotherapy use is also more prevalent among non-formulary drug claims (this was expected for Saxenda) relative to the PT drug plans (Table 5). For comparison, 17.2% of Ozempic claimants used the drug as monotherapy in Ontario in 2021, whereas this proportion ranged from 39.9% (in Manitoba) to 68.2% (in British Columbia) among the 3 provinces included in the non-formulary claims data. The proportion of monotherapy use among non-formulary GLP-1 RA claimants has increased year over year, especially with Ozempic, Rybelsus, Trulicity, and Victoza, and will likely continue to rise, similar to what is occurring among the PT drug plans. These findings are in line with what was observed when estimating suspected non-diabetic use based on prior claims history.
Table 5
GLP-1 RA Monotherapy Claimants by Jurisdiction, Year, and Drug Among Non-Formulary Claims (Percentage of GLP-1 Claimants).
As noted in Appendix 3, Ozempic in T2DM is covered by public formularies after treatment with MET alone or MET in combination with a sulfonylurea or insulin is shown to be insufficient for glycemic management. In all cases, MET is stipulated as first-line therapy. Accordingly, among PT public drug plans, MET was the most frequently co-prescribed medication in combination with Ozempic (74.5% of combination therapy Ozempic claimants) in 2021 (Figure 4). Other antidiabetic medications prescribed in combination with Ozempic across these formularies in 2021 were (in diminishing order of frequency): SGLT2 inhibitors (56.2%), insulin (49.0%), sulfonylureas (28.4%), and DPP-4 inhibitors (12.0%); there were substantially lower frequencies for repaglinide (0.7%), acarbose (0.6%), and thiazolidinediones (0.4%).
A breakdown of the utilization of Ozempic-containing combination therapies by jurisdiction across public PT formularies in 2021 is presented in Appendix 8. In summary, combination therapy with MET is consistently prevalent across jurisdictions (ranging from 70.3% in New Brunswick to 87.7% in Newfoundland and Labrador), along with the concurrent use of insulin (ranging from 37.7% in Newfoundland and Labrador to 54.9% in Prince Edward Island). Rates of SGLT2 inhibitor combination therapy use varies widely across jurisdictions; the highest rate was in Ontario (60.5%) and there were much lower rates in the Atlantic provinces (New Brunswick: 11.9%; Nova Scotia: 8.1%; Prince Edward Island: 6.3%; Newfoundland and Labrador: 3.1%). It is also notable that SGLT2 inhibitors are listed as Open Benefit in the Ontario public formulary (with the exception of Steglatro).30 Combination with a SU also varies across jurisdictions, ranging from 19.5% (Alberta) to 87.7% (Newfoundland and Labrador). DPP-4 inhibitor use varies widely across jurisdictions; the highest rates occur in Ontario (14.1%) and Alberta (12.3%), with negligible rates in British Columbia (0.9%), Newfoundland and Labrador (0.0%), and Nova Scotia and Prince Edward Island (0.3% each).

Figure 4
Antidiabetic Drugs Used With Ozempic in Combination Therapies Across Public PT Formularies Nationally in 2021 (Percentage of Ozempic Combination Therapy Claimants).
Ozempic Dosing Utilization (Approach 3)
Utilization of Ozempic by dosing (mg/week) and by jurisdiction is provided in Appendix 9. This results of this were that the majority of Ozempic claimants in 2021 (more than 90% across all jurisdictions; 96.4% nationally) received the drug at 1 mg or less weekly, which is in line with the original maintenance dose for T2DM according to the product monograph before it was updated with new dosing in January 2022.
Drug Expenditures on GLP-1 RAs
Since 2016, more than $357 million has been spent on GLP-1 RAs across all public PT formularies (Appendix 10). Although expenditures on Adlyxine, Ozempic, and Saxenda began in 2019, spending on Ozempic has represented the majority of these expenditures to date. Of the more than $357 million in total GLP-1 RA expenditures, only approximately $113 thousand (or 0.03% of this spending) has been spent on Adlyxine, Saxenda, and Victoza, while the rest was spent on Ozempic; therefore, the total increase in national GLP-1 RA spending by public PT formularies has been driven by Ozempic. There was more than a 700% increase in GLP-1 RA spending from 2019 to 2020, and another 94% increase from 2020 to 2021 (Figure 5). If this trend continues, it is projected that another $333 million will be spent on GLP-1 RAs in 2022, which would equate to another 47% increase from 2021 to 2022.
Additionally, because of the increasing percentage of suspected non-diabetic (based on prior claims history) Ozempic claimants from 2019 to 2021, the percentage of drug expenditures on Ozempic attributed to such use has also increased from 4.2% in 2019, to 6.2% in 2020, and 10.1% in 2021 (Figure 6).

Figure 5
National Public Drug Plan Expenditures on GLP-1 RAs by Year From 2019 to 2022 (Projected).
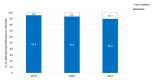
Figure 6
Percentage of National Public Provincial and Territorial Drug Plan Expenditures on Ozempic Attributed to Diabetic and Suspected Non-Diabetic Use (Based on Prior Claims History) Since 2019.
Discussion
Overall trends and national expenditures: Of the 6 GLP-1RAs currently approved by Health Canada for use in T2DM, only 2 are currently reimbursed through public formularies: Adlyxine and Ozempic. Ozempic has more extensive coverage relative to Adlyxine; it is listed on every public drug plan formulary. Ozempic is listed as Open Benefit in Ontario and the CSC, NIHB, and VAC, and with restrictions (prior approval or special authorization) in every other jurisdiction. Therefore, Ontario drives the market share for Ozempic utilization across the public PT formularies, while the market shares for other GLP-1 RAs are more evenly distributed across non-formulary drug claims (Figure 1 and Figure 2), although Ozempic still makes up the greatest GLP-1 RA market share among non-formulary claimants and is increasing year over year (from 21.5% in 2018 to 69.0% in 2021). Across all drug plans, year-to-year increases in GLP-1 RA claims are unambiguous and evident. Total national expenditures on GLP-1 RAs across all public PT formularies since 2016 now stand at more than $357 million and are expected to continue to grow rapidly (Figure 5). Only approximately $113,000 (0.03%) has been spent on Adlyxine, Saxenda, and Victoza, while the rest was spent on Ozempic since reimbursement began in 2019. Because these expenditures are specific to public PT drug plans, it is expected that costs considering federal drug plan, out-of-pocket, and private drug plan expenditures on these medications would add substantially to this $357 million figure.
Suspected non-diabetic use of Ozempic: Given that there is evidence suggesting that GLP-1 RAs may be used for other indications outside of T2DM (e.g., weight loss and cardiovascular disease),20-22 many drug plans were interested in understanding the current utilization of these medications under their current listing status and reimbursement criteria. Using the claims-based approach of previous use of diabetes medications or glucose monitoring devices (i.e., approach 1), the estimates for suspected non-diabetic use have increased year over year, from 4.2% in 2019, 6.9% in 2020, to 10.7% of all Ozempic claimants nationally in 2021 (Table 2) — and is projected to increase in 2022. When comparing jurisdictions, it was determined that the frequency of suspected non-diabetic use of Ozempic appears to be associated with its listing status and reimbursement criteria in each jurisdiction. The proportion of claimants using Ozempic for suspected non-diabetic use (based on prior claims history) was highest in Ontario, where Ozempic is an Open Benefit, and New Brunswick and Alberta, where their reimbursement criteria specifically state that individuals who are intolerant or have a contraindication to MET are eligible for Ozempic. The year-to-year trend in the percentage of PT drug plan expenditures attributed to suspected non-diabetic use matches closely matches the results of the analysis on unique claimants (Figure 6). These figures represent a possible upper limit of estimated use of Ozempic for weight management or any other indication other than T2DM. Note that this analysis from public formularies does not provide any indication of prior claims for other antidiabetic drugs or glucose monitoring that were covered by private insurance plans, which may be a factor when determining a claimant’s prior drug claims history (i.e., other antidiabetic drugs or glucose monitoring may have been covered by a claimant’s private insurance plan) and could overestimate the suspected non-diabetic use of Ozempic. However, this method could actually underestimate the frequency of suspected non-diabetic use because individuals may be filling prescriptions for other antidiabetic medications and not taking them to circumvent the reimbursement criteria (e.g., the minimum dose and days’ supply of MET is claimed by an individual who does not have T2DM and who has no intention of taking the drug, so they can be granted access to Ozempic); this is an area for future research.
The analysis on non-formulary drug claimants was limited to 3 provinces (British Columbia, Manitoba, and Saskatchewan) because these were the only data available to CIHI and these data include claims paid by cash or covered by private insurance or a federal drug plan. Federal plans were informally surveyed; it was estimated that approximately one-third of all these non-formulary claimants were likely covered by a federal plan, thus the remaining portion of claimants were estimated to be individuals who were paying out-of-pocket or were privately insured. If we assume that GLP-1 RAs are an Open Benefit among most private drug plans, it stands to reason that the suspected non-diabetic use of these medications would be higher among non-formulary claimants (which was indeed the case). More research is needed on private payer data to estimate how often GLP-1 RAs are being used off-criteria.
Even with the concerns previously mentioned about this method (i.e., approach 1), it is still likely the best estimate of suspected non-diabetic Ozempic use relative to the other approaches. Determining the proportion of claimants using Ozempic monotherapy (approach 2) is more likely to overestimate suspected non-diabetic use because individuals living with T2DM who have a contraindication or intolerance to other antidiabetic medications can use Ozempic monotherapy. Approach 3 based on Ozempic dosing (i.e., high versus low dose) is limited for 2 reasons: dosing was estimated based on other parameters included in the claims data and not on a patient’s actual dose regimen (refer to Limitations section) and there is the possibility that patients using Ozempic for a non-diabetic indication are using it at a lower dose (i.e., similar to the dosing regimen for the T2DM indication before January 2022) so dose-escalation can be dependent on a patient’s response to treatment and, if this was the case, this particular analysis would provide no indication of potential non-diabetic use. Suspected non-diabetic use of Ozempic based on prior claims history (approach 1) is expected to be more accurate based on the indication listed in the product monograph and current reimbursement criteria for Ozempic across public drug plans.
The additional analysis on continued suspected non-diabetic use in the look-forward period after the index GLP-1 RA claim revealed that 70.5% of these claims were projected to still be for suspected of non-diabetic use. These findings can be explained by any, or a combination of any, the following reasons:
- claims made for other antidiabetic medications or glucose monitoring for a given individual suspected of non-diabetic GLP-1 RA use were not captured in the 2-year look-back period (i.e., some of these individuals were living with T2DM but their claims for other antidiabetic medications or glucose monitoring were made more than 2 years before the index GLP-1 RA claim)
- individuals suspected of non-diabetic GLP-1 RA use did not have DM but eventually developed T2DM following their index GLP-1 RA claim
- individuals suspected of non-diabetic GLP-1 RA use actually did have DM but started filling prescriptions for other antidiabetic medications or glucose monitoring devices due to a lack of glycemic management on GLP-1 RA monotherapy.
Ozempic Monotherapy and Use in Combination Therapy
Overall, the national use of Ozempic as monotherapy (as a proportion of all Ozempic claimants) from 2019 to 2021 was fairly consistent, with a decrease from 15.8% in 2019 to 11.8% in 2020 and an increase to 16.8% in 2021 (Table 4). The figures for Ozempic monotherapy are considerably higher than the suspected non-diabetic use estimates based on prior claims history. Note that some of these individuals may be living with T2DM and are using Ozempic as monotherapy due to a contraindication or intolerance to other antidiabetic medications. In the case of MET, contraindications include chronic renal impairment, chronic liver disease, myocardial infarction, and cardiac failure. The potential risk of lactic acidosis with MET in individuals who have contraindications for its use has been shown to be largely exaggerated.32-38 Real-world prescription practices typically aim to prescribe MET regardless of any contraindications based on the benefits outweighing the risks. Estimates of the proportions of individuals prescribed MET despite contraindications range from 25%39 to 80%40; it has also been estimated that a small percentage (6.4%) of individuals living with T2DM actually have a contraindication to MET.39
In combination therapy, MET, SGLT2 inhibitors, insulin, and sulfonylureas were the 4 leading antidiabetic medications concomitantly prescribed with Ozempic (Figure 4). These are the treatments for which Ozempic is indicated according to the Health Canada–approved product label12; therefore, the presence of these other drugs as the most frequently co-prescribed medications is not surprising, although it is surprising for SGLT2 inhibitors to be higher than insulin and sulfonylureas, which have been the mainstays of T2DM treatment for decades. In support of increased use of SGLT2 inhibitors in combination with GLP-1 RAs, recent systematic literature reviews and meta-analyses have confirmed the benefits in increased safety and efficacy along with renal protective benefits.41,42 There is a 4-fold lower use of DPP-4 inhibitors compared with SGLT2 inhibitors in combination with Ozempic (Figure 4), but the combination of GLP-1 RA and DPP-4 inhibitor is not recommended.43,44 This is supported by a recent systematic literature review,45 a case series,45 and a review of head-to-head clinical trials.46 Additional research is required to investigate why GLP-1 RAs and DPP-4 inhibitors are being used in combination at such a high proportion, especially in jurisdictions with less restrictive access.
Limitations
The current analyses have several limitations that warrant highlighting:
- The claims data from NPDUIS were not indication-specific. Thus, different strategies were used to separate Ozempic use in T2DM from suspected use in other indications. Dosing data were used in 1 strategy to estimate suspected non-diabetic use because the weight management indication had different dosing than the T2DM indication before January 2022. Claims data with no prior antidiabetic treatment or accompanying glucose monitoring device claims were also used. This approach cannot account for any claims made on private health insurance plans concomitantly with public plans. The analyses presented here did not take into account the clinical rationale for treatments. Physicians may prescribe based on a combination of clinical sensitivity to the patients’ needs, their eligibility for reimbursement through private insurance, or other coverage criteria and access. The analyses did not provide any insight on a physician’s decision to prescribe (e.g., Ozempic for weight loss).
- The 2-year “look-back” period for prior antidiabetic medications or glucose monitoring may miss some prior claims before that period. If prior claims beyond that period were missed, and the individual switched to private benefits or simply stopped making claims for antidiabetic medications or glucose monitoring devices before this look-back period, the suspected non-diabetic use of Ozempic would be overestimated. This overestimation could also vary from jurisdiction-to-jurisdiction based on how their drug plan operates (e.g., residents in Ontario are generally covered by private insurance until they reach 65 years of age).
- Data from non-formulary claims were limited to British Columbia, Manitoba, and Saskatchewan, which excludes a potentially large pool of claims from federal drug plans and private insurance elsewhere in Canada. This is especially important when evaluating the use of GLP-1 RAs nationally and their financial impact on the entire country across both public and private payers.
- The analysis based on dosing was associated with some degree of uncertainty because dosing was estimated based on other parameters (i.e., quantities and total supply days) and not on the individual’s actual dosing regimen. For example, it is difficult to calculate doses of Ozempic because it is administered using multidose pens, which are available at different doses. Additionally, adherence to the medication plays a role in the frequency of prescription fills and the medication may not be taken at the prescribed dose. The total supply days variable is not a reliable metric because it is based on manual entry at the pharmacy level and there is a degree of error or potentially inaccurate data entry associated with this process. Dosing may be personalized depending on the occurrence of gastrointestinal events; therefore, in this scenario, dosing is reflective of the individual’s intolerance to the drug as opposed to its indication for use. Lastly, individuals may request multiple pens at the same time if they are travelling or they may move between provinces, which would misrepresent the dose at which the medication was actually prescribed.
Conclusions and Implications for Decision- or Policy-Making
Drug plans spend a significant amount on GLP-1 RAs, most notably Ozempic, and these expenditures are growing at a rapid rate. This may be partly attributed to the off-criteria use of these medications outside of T2DM, which appears to be associated with drug plan listing status and reimbursement criteria. The trend in suspected non-diabetic GLP-1 RA use and its associated expenditures are increasing year-to-year across public drug plans, which warrants formulary management strategies to promote their appropriate use. Use of medications off-criteria undermines the intended reimbursement of therapies, and may result in less value for money for payers. The utilization analysis also showed that less restrictive drug listing and reimbursement criteria can also lead to clinically inappropriate combination regimens (e.g., GLP-1 RA plus DPP-4 inhibitors), which increases the cost of treatment at no added benefit. Formulary management strategies can include (but are not limited to) prior approval or authorization for GLP-1 RA reimbursement or audits of pharmacy dispensing practices. Future research in this area includes assessing the utilization trends with private insurance, determining the degree to which individuals may be circumventing existing reimbursement criteria, and assessing the impact of direct-to-consumer advertising in drug utilization.
Abbreviations
- CDEC
CADTH Canadian Drug Expert Committee
- CIHI
Canadian Institute for Health Information
- DM
diabetes mellitus
- DPP-4
dipeptidyl peptidase-4
- GLP-1
glucagon-like peptide-1
- NIHB
Non-Insured Health Benefits
- NPDUIS
National Prescription Drug Utilization Information System
- PT
provincial and territorial
- RA
receptor agonist
- SC
subcutaneous
- SGLT2
sodium-glucose cotransporter-2
- T2DM
type 2 diabetes mellitus
Acknowledgements: Roger Cheng, Trupti Jani, Canadian Institute for Health Information (CIHI), Colleen Metge.
References
- 1.
- LeBlanc AG, Jun Gao Y, McRae L, Pelletier C. At-a-glance - Twenty years of diabetes surveillance using the Canadian Chronic Disease Surveillance System. Health Promot Chronic Dis Prev Can. 2019;39(11):306-309. [PMC free article: PMC6876649] [PubMed: 31729313]
- 2.
- Diabetes Canada. Type 2 Diabetes. https://www
.diabetes .ca/about-diabetes/type-2. Published 2022. Accessed July 27, 2022. - 3.
- Diabetes Canada Clinical Practice Guidelines Expert C, Houlden RL. Introduction. Can J Diabetes. 2018;42 Suppl 1:S1-S5. [PubMed: 29650079]
- 4.
- Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999-1008. [PMC free article: PMC4159698] [PubMed: 24084292]
- 5.
- Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559. [PMC free article: PMC4551392] [PubMed: 18539917]
- 6.
- Drucker DJ, Goldfine AB. Cardiovascular safety and diabetes drug development. Lancet. 2011;377(9770):977-979. [PubMed: 21316097]
- 7.
- Ferro EG, Elshazly MB, Bhatt DL. New Antidiabetes Medications and Their Cardiovascular and Renal Benefits. Cardiol Clin. 2021;39(3):335-351. [PubMed: 34247748]
- 8.
- Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102. [PMC free article: PMC8085572] [PubMed: 33068776]
- 9.
- AstraZeneca Canada Inc. Product information for Byetta. https://www
.astrazeneca .ca/content/dam/az-ca /downloads/productinformation /byetta-product-monograph-en.pdf. Published 2019. Accessed August 2, 2022. - 10.
- AstraZeneca Canada Inc. Product information for Bydureon. https://www
.astrazeneca .ca/content/dam/az-ca /downloads/productinformation /bydureon-product-monograph-en.pdf. Published 2020. Accessed August 2, 2022. - 11.
- Eli Lilly Canada Inc. Product information for Trulicity. https://pi
.lilly.com/ca/trulicity-ca-pm .pdf. Published 2021. Accessed August 2, 2022. - 12.
- Novo Nordisk Canada. Product information for Ozempic. https://www
.novonordisk .ca/content/dam/nncorp /ca/en/products /ozempic-product-monograph.pdf. Published 2022. Accessed August 2, 2022. - 13.
- Novo Nordisk Canada Inc. Product information for Victoza. https://www
.novonordisk .ca/content/dam/Canada /AFFILIATE/www-novonordisk-ca /OurProducts /PDF/victoza-product-monograph.pdf. Published 2020. Accessed August 2, 2022. - 14.
- Novo Nordisk Canada Inc. Product information for Rybelsus. . https://www
.novonordisk .ca/content/dam/Canada /AFFILIATE/www-novonordisk-ca /OurProducts /PDF/Rybelsus-PM-EN-monograph.pdf. Published 2020. Accessed August 2, 2022. - 15.
- Sanofi-Aventis Canada Inc. Product information for Adlyxine. https://products
.sanofi .ca/en/adlyxine.pdf. Published 2020. Accessed August 2, 2022. - 16.
- Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19(4):524-536. [PubMed: 27981757]
- 17.
- Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18(3):203-216. [PMC free article: PMC4785614] [PubMed: 26489970]
- 18.
- Novo Nordisk Canada Inc. Product information for Saxenda. https://www
.novonordisk .ca/content/dam/nncorp /ca/en/products /saxenda-product-monograph.pdf.pdf. Published 2021. Accessed August 2, 2022. - 19.
- Novo Nordisk Canada Inc. Product information for Wegovy. https://www
.novonordisk .ca/content/dam/nncorp /ca/en/products /Wegovy-product-monograph.pdf. Published 2022. Accessed August 2, 2022. - 20.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA. 2015;314(7):687-699. [PubMed: 26284720]
- 21.
- Singh G, Krauthamer M, Bjalme-Evans M. Wegovy (semaglutide): a new weight loss drug for chronic weight management. J Investig Med. 2022;70(1):5-13. [PMC free article: PMC8717485] [PubMed: 34706925]
- 22.
- Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. [PMC free article: PMC3256253] [PubMed: 22236411]
- 23.
- CADTH. Liraglutide (Saxenda) - Reimbursement Review. https://www
.cadth.ca /sites/default/files /DRR/2021/SR0668%20Saxenda %20-%20CADTH%20Final %20Rec%20KT_BF_KT-pw.pdf. Published 2021. Accessed August 4, 2022. - 24.
- CADTH. Semaglutide (Wegovy) - Reimbursement Review. https://www
.cadth.ca/semaglutide-1. Published 2022. Accessed August 4, 2022. - 25.
- Statistics Canada. Health Fact Sheets - Overweight and obese adults, 2018. https://www150
.statcan .gc.ca/n1/pub/82-625-x /2019001/article/00005-eng.htm. Published 2018. Accessed August 6, 2022. - 26.
- Courtney Greenberg. Type 2 diabetes drug being used for weight loss leads to shortage in Australia. National Post. May 31, 2022.
- 27.
- Kolovos B. Shortage of diabetes medication Ozempic after TikTok users promote drug for weight loss. The Guardian. May 31, 2022.
- 28.
- Therapeutic Goods of Australia. Joint statement: Prioritisation of semaglutide (Ozempic) supply for people with type 2 diabetes during shortage. https://www
.tga.gov.au /alert/joint-statement-prioritisation-semaglutide-ozempic-supply-people-type-2-diabetes-during-shortage. Published 2022. Accessed August 10, 2022. - 29.
- Health product advertising incidents. 2022. https://www
.canada.ca /en/health-canada/services /drugs-health-products /regulatory-requirements-advertising /health-product-advertising-complaints.html. Accessed August 10, 2022. - 30.
- Diabetes Canada. Formulary Listings for Diabetes Medications in Canada. 2021.
- 31.
- CADTH. Semaglutide (Ozempic) - Reimbursement Review. https://www
.cadth.ca /sites/default/files /cdr/complete/SR0594 %20Ozempic%20-%20CDEC %20Final%20Recommendation %20May%2017%2C %202019%20%28redacted%29_For%20posting .pdf. Published 2019. Accessed August 4, 2022. - 32.
- Holstein A, Nahrwold D, Hinze S, Egberts EH. Contra-indications to metformin therapy are largely disregarded. Diabet Med. 1999;16(8):692-696. [PubMed: 10477216]
- 33.
- Holstein A, Stumvoll M. Contraindications can damage your health--is metformin a case in point? Diabetologia. 2005;48(12):2454-2459. [PubMed: 16283245]
- 34.
- Huang W, Castelino RL, Peterson GM. Metformin usage in type 2 diabetes mellitus: are safety guidelines adhered to? Intern Med J. 2014;44(3):266-272. [PubMed: 24405767]
- 35.
- Jones GC, Macklin JP, Alexander WD. Contraindications to the use of metformin. BMJ. 2003;326(7379):4-5. [PMC free article: PMC1124930] [PubMed: 12511434]
- 36.
- Lu WR, Defilippi J, Braun A. Unleash metformin: reconsideration of the contraindication in patients with renal impairment. Ann Pharmacother. 2013;47(11):1488-1497. [PubMed: 24259604]
- 37.
- McCormack J, Johns K, Tildesley H. Metformin's contraindications should be contraindicated. CMAJ. 2005;173(5):502-504. [PMC free article: PMC1188187] [PubMed: 16129871]
- 38.
- Sweileh WM. Contraindications to metformin therapy among patients with type 2 diabetes mellitus. Pharm World Sci. 2007;29(6):587-592. [PubMed: 17333496]
- 39.
- Emslie-Smith AM, Boyle DI, Evans JM, Sullivan F, Morris AD, Collaboration DM. Contraindications to metformin therapy in patients with Type 2 diabetes--a population-based study of adherence to prescribing guidelines. Diabet Med. 2001;18(6):483-488. [PubMed: 11472468]
- 40.
- Pongwecharak J, Tengmeesri N, Malanusorn N, Panthong M, Pawangkapin N. Prescribing metformin in type 2 diabetes with a contraindication: prevalence and outcome. Pharm World Sci. 2009;31(4):481-486. [PubMed: 19462255]
- 41.
- Guo M, Gu J, Teng F, et al. The efficacy and safety of combinations of SGLT2 inhibitors and GLP-1 receptor agonists in the treatment of type 2 diabetes or obese adults: a systematic review and meta-analysis. Endocrine. 2020;67(2):294-304. [PubMed: 31900793]
- 42.
- Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845-854. [PubMed: 31495651]
- 43.
- Gallwitz B. Clinical Use of DPP-4 Inhibitors. Front Endocrinol (Lausanne). 2019;10:389. [PMC free article: PMC6593043] [PubMed: 31275246]
- 44.
- Geurin MD. Drug Combo Adds No Benefit in Patients with Type 2 Diabetes. Am Fam Physician. 2016;93(6):436-438. [PubMed: 26977827]
- 45.
- Lajthia E, Bucheit JD, Nadpara PA, et al. Combination therapy with once-weekly glucagon like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a case series. Pharm Pract (Granada). 2019;17(4):1588. [PMC free article: PMC6935552] [PubMed: 31897252]
- 46.
- Gilbert MP, Pratley RE. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front Endocrinol (Lausanne). 2020;11:178. [PMC free article: PMC7145895] [PubMed: 32308645]
Appendix 1. GLP-1 RA Products Approved in Canada
Note that this appendix has not been copy-edited.
Table 6
GLP-1 RA Products Approved in Canada.
Appendix 2. Dosing For GLP-1 RAs (Administered Subcutaneously) Indicated for Both T2DM and Weight Management
Note that this appendix has not been copy-edited.
Table 7
Dosing for GLP-1 RAs (Administered Subcutaneously) Indicated for Both T2DM and Weight Management.
Appendix 3. Funding Criteria for GLP-1 RAs by Jurisdiction Current as of July 29, 2022
Note that this appendix has not been copy-edited.
Table 8
Funding Criteria for GLP-1 RAs by Jurisdiction as of July 29, 2022.
Appendix 4. List of Public Drug Plans and Programs Included in Utilization Analysis
Note that this appendix has not been copy-edited.
Provincial public drug plans and programs with claims data contained within the NPDUIS database within the requested [between January 1, 2016, and December 31, 2021] time period
Table 9
List of Public Drug Plans and Programs Included in Utilization Analysis.
Appendix 5. Drugs Included in the National Prescription Drug Utilization System Database Search
Note that this appendix has not been copy-edited.
Table 10
Drugs Included in the National Prescription Drug Utilization System Database Search.
Appendix 6. Analytical Methods
Note that this appendix has not been copy-edited.
No statistical analyses were planned for this study. There were also no minimal clinically important difference thresholds to consider for this study. The analyses performed for this study were descriptive in nature and conducted to assess trends and numerical differences in the utilization of and total expenditures on GLP-1 RAs across Canada, considering both public and private drug plans.
Reimbursement Criteria
To determine reimbursement criteria across federal, provincial, and territorial drug plans, formulary websites and documents containing lists of regular benefit and restricted access drugs were searched. The reimbursed formulations, criteria, and any restrictions and notes were summarized for all federal, provincial, and territorial drug plans except Quebec. For each GLP-1 RA, it was determined whether the product is listed for reimbursement on the drug plan’s formulary. If listed, it was also determined whether the product is listed as an open benefit or if a more restrictive review process (e.g., special authorization) is required for reimbursement. The listing statuses and reimbursement criteria of GLP-1 RAs across all public drug plans were tabulated and summarized qualitatively.
Utilization Patterns
Utilization patterns were based on the number of total claimants of GLP-1 RAs through public drug programs, by jurisdiction, calendar year, brand name, ATC level 5 codes/description, and dose category (for Victoza and Ozempic), from 2016 to 2021. Market shares for each drug were calculated as a proportion of all GLP-1 RA claimants. In cases of suppressed data (i.e., values with less than 5 claimants, but greater than 0), it was assumed that 2 claimants made a claim for the drug. These calculations were performed within each jurisdiction and at the national level across both formulary and non-formulary drug claims data.
Suspected Non-Diabetic Use of GLP-RAs
Suspected non-diabetic use among GLP-1 RA claimants was estimated using 3 different approaches:
- The proportion of GLP-1 RA claimants without prior antidiabetic drug or glucose monitoring device claims within a 2-year look-back period from the index GLP-1 RA claim.
- If claimants had a prior claim for another antidiabetic drug or glucose monitoring device, they were categorized as “yes” in terms of being a patient with T2DM; otherwise, they were categorized as “no.”
- Claimants categorized as “no” with less than 6 months of prior claims history were excluded from this analysis and not included as suspected non-diabetic GLP-1 RA claimants. This threshold was selected as patients with T2DM will generally refill their prescriptions within a 6-month period.
- An additional analysis of claimants deemed to not have DM using this definition was also conducted to determine if they continued not to make claims for another antidiabetic drug or glucose monitoring device (i.e., continued non-diabetic use) in the look-forward period (i.e., any time after the index GLP-1 RA claim).
- The proportion of GLP-1 RA claimants using the GLP-1 RA as monotherapy.
- Concurrent use of other antidiabetic medications was defined as claims made another antidiabetic drug after the GLP-1 RA index date and prior to a subsequent claim for the same GLP-1 RA. If no claim for another antidiabetic medication was made within this period, the claimant was defined as a patient using GLP-1 RA monotherapy (i.e., suspected non-diabetic use unless the patient had a contraindication or intolerance to other antidiabetic drugs).
- The proportion of Victoza and Ozempic claimants using these medications at various dose regimens.
- Prior to January 2022, the maintenance doses for both Victoza (1.8 mg daily) and Ozempic (1.0 mg weekly) were about half that of their weight management formulations (Saxenda [3.0 mg daily] and Wegovy [2.4 mg weekly], respectively). As of January 2022, as higher maintenance dose (2.0 mg weekly) was approved for Ozempic for T2DM. This analysis categorized GLP-1 RA claimants into different dosing categories considering the above.
- This analysis was based on quantities claimed and total supply days. This analysis was not feasible for Victoza due to limited claims data for this product. Ozempic claimants were categorized under the following 5 dosing categories:
- ≤ 1 mg weekly
- > 1 and ≤ 1.3 mg weekly
- > 1.3 and ≤ 2 mg weekly
- > 2 and ≤ 4 mg weekly
- > 4 mg weekly
These analyses were conducted for each drug, by year from 2016 to 2021, within each jurisdiction and at the national level. When applicable, estimates for 2022 were projected using linear regression via the FORECAST.LINEAR function in Microsoft Excel. This analysis was conducted using public formulary claims data only as dosing data related to non-formulary claims were not available.
Drug Expenditures
Expenditures were based on the total prescription cost accepted by the drug plan. Total expenditures were calculated for each drug, by year from 2016 to 2021, within each jurisdiction and at the national level. In cases of suppressed data, expenditures were estimated using national cost per claimant estimates calculated from the available data. Cost shares for each drug were calculated as a proportion of total GLP-1 RA expenditures. When applicable, estimates for 2022 were projected using linear regression via the FORECAST.LINEAR function in Microsoft Excel. Year-to-year percent increases in expenditures were also calculated, as was the proportion of spending attributed to non-diabetic use. This analysis was conducted using public formulary claims data only as expenditure data related to non-formulary claims were not available.
Table 11
Other Antidiabetic Medications.
Table 12
Glucose Monitoring Systems.
Appendix 7. Data Elements Provided in the Dataset and Their Definitions in Terms of Claims Information
Note that this appendix has not been copy-edited.
Table 13
Data Elements Provided in the Dataset and Their Definitions in Terms of Claims Information.
Appendix 8. Ozempic Dosing Utilization by Jurisdiction in 2021 (Percentage of Ozempic Claimants)
Table 14
Ozempic Dosing Utilization by Jurisdiction in 2021 (Percentage of Ozempic Claimants) .
Appendix 9. Ozempic Combination Therapies by Jurisdiction Across Public Formularies in 2021 (Percentage of Ozempic Combination Therapy Claimants)
Table 15
Ozempic Combination Therapies by Jurisdiction Across Public Formularies in 2021 (Percentage of Ozempic Combination Therapy Claimants).
Appendix 10. National Expenditures on GLP-1 RAs by Year Across Public Provincial and Territorial Drug Plans
Note that this appendix has not been copy-edited.
Table 16
National Expenditures on GLP-1 RAs by Year Across Public Provincial and Territorial Drug Plans.
The views expressed in this article are the authors’ and do not necessarily represent the views of the Canadian Institute for Health Information.
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.
The copyright and other intellectual property rights in this document are owned by CADTH and its licensors. These rights are protected by the Canadian Copyright Act and other national and international laws and agreements. Users are permitted to make copies of this document for non-commercial purposes only, provided it is not modified when reproduced and appropriate credit is given to CADTH and its licensors.
About CADTH: CADTH is an independent, not-for-profit organization responsible for providing Canada’s health care decision-makers with objective evidence to help make informed decisions about the optimal use of drugs, medical devices, diagnostics, and procedures in our health care system.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
- Summary
- Background
- Purpose of This Analysis
- Policy Issues
- Methods
- Findings
- Discussion
- Limitations
- Conclusions and Implications for Decision- or Policy-Making
- Abbreviations
- References
- GLP-1 RA Products Approved in Canada
- Dosing For GLP-1 RAs (Administered Subcutaneously) Indicated for Both T2DM and Weight Management
- Funding Criteria for GLP-1 RAs by Jurisdiction Current as of July 29, 2022
- List of Public Drug Plans and Programs Included in Utilization Analysis
- Drugs Included in the National Prescription Drug Utilization System Database Search
- Analytical Methods
- Data Elements Provided in the Dataset and Their Definitions in Terms of Claims Information
- Ozempic Dosing Utilization by Jurisdiction in 2021 (Percentage of Ozempic Claimants)
- Ozempic Combination Therapies by Jurisdiction Across Public Formularies in 2021 (Percentage of Ozempic Combination Therapy Claimants)
- National Expenditures on GLP-1 RAs by Year Across Public Provincial and Territorial Drug Plans
- Current Utilization Patterns of Glucagon-Like Peptide-1 Receptor AgonistsCurrent Utilization Patterns of Glucagon-Like Peptide-1 Receptor Agonists
Your browsing activity is empty.
Activity recording is turned off.
See more...
